Why to use fuel additives
Fuel producers adhere to quality norms set by the law. Currently, the minimum diesel cetane number is 51 and petrol octane number is 95.
Increasing these numbers will improve fuel efficiency, lower the emissions and therefore it will also lower your fuel expenses. You can see the example in the chart below:
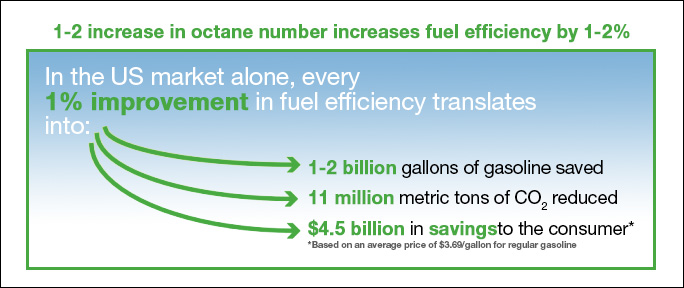
Apart from increasing the octane and cetane number, our CAR PILLS help to protect the engine – by reducing the friction and better combustion, they help to maintain the motor and the fuel system cleaner.
Cetane Improvement
Car Pills as a cetane improver of diesel fuel aid in start ability especially on cold mornings by reducing the diesel fuel ignition delay time in the combustion chamber. Car Pills fuel additive increases the combustibility of low and mid-range diesel fuels by enhancing complete combustion thus reducing smoke emissions, unburned hydrocarbons (CO) and odour.
Stabilizer and anti-oxidant
Distillate (diesel) fuels are organic in nature and from the time of manufacture (refining) until they are used diesel fuels will degrade. Car Pills additive retard the natural hydrocarbon degradation of diesel fuels and distillate fuel oils during long-term storage.
Cleaner
Car Pills advanced additive is used as cleaners to remove carbon and gummy deposits from the diesel fuel delivery and injection systems and keep the entire system clean to ensure a proper diesel fuel spray pattern. This ensures more complete combustion and increases the power produced.
Lubricity
Light duty rotary diesel fuel injection pumps rely on the diesel fuel for lubrication. Lubricity is the diesel fuels ability to reduce friction in moving parts in diesel fuel pumps and diesel fuel injectors. Today’s Ultra Low Sulfur diesel fuels reduce the natural lubrication in the diesel fuel. Diesel fuel lubricity additives increase the diesel fuels ability to reduce friction in moving parts in diesel fuel pumps and diesel fuel injectors.
Graphene
Graphene, a single layer of carbon atoms closely packed into honeycomb two-dimensional (2D) lattice, has attracted enormous attention recently. Its hybrid carbon network, as well as extraordinary electronic, thermal and mechanical properties, are interesting for a wide range of applications. Graphene was one of the most expensive nano-particles in the world.
Graphene as Nano Lubricant
Scientists discovered that they could substitute one-atom-thick graphene layers for either solid- or oil-based lubricants on sliding steel surfaces, enabling a dramatic reduction in the amount of wear and friction. Reducing energy and materials losses in these moving mechanical systems due to friction and wear remains one of the greatest engineering challenges of our time.
The graphene was found to adhere strongly to the surface during testing. Even partial coatings are very effective at reducing friction because of the ability of the graphene to reorient itself during initial wear cycles and can last a considerable length of time providing low friction during sliding.
Benefits of graphene as a lubricant:
- Superlubricity with no measurable wear of extended time
- Near zero friction
- Works in a dry and humid environment
- Virtually eliminates friction and wear
Power, fuel efficiency
Independent Engine Dyno Testing Without and With Diesel Fuel Additive:
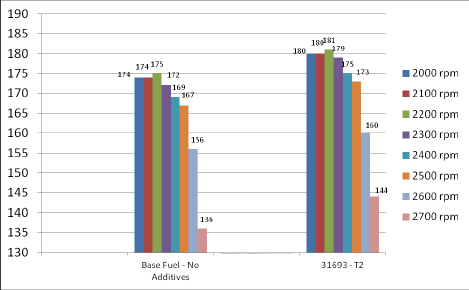
Brake Horsepower Increases at Various Engine RPM with Nano Fuel Additive
Subsequent engine efficiency improvements have been well documented in large fleet use.
Field trials have consistently demonstrated fuel efficiency improvements of 3-5% for slow-medium speed types of diesel, 5-8% for high-speed types of diesel.
Test methods employed were:
- Specific fuel consumption.
- Exhaust Carbon Balance.
- Statistical Computer Model.
The Exhaust Carbon Balance method (AS2077-1982) offers the most accurate, quick and hassle-free test method. This is because it measures instantaneous flow rates. The above results have been confirmed in controlled trials at Curtin University’s thermo-dynamics laboratory (WA). Quite simply, less fuel is required to do the same work, and more power is produced for the same throttle setting. This is because it measures instantaneous flow rates. The above results have been confirmed in controlled trials at Curtin University’s thermo-dynamics laboratory (WA). Quite simply, less fuel is required to do the same work, and more power is produced for the same throttle setting.
Picrate
Strictly speaking, the action of picrate is not chemical. That is, it does not alter fuel specifications, and will not provide more energy than contained in the fuel, so there can be no detrimental effects. This is important from an engine warranty point of view.
The overall reaction is modified to favour oxidation reactions. More complete combustion is achieved in a shorter time. The action is threefold. Primary atomisation. The solvent carrier acts as a misting agent upon injection of the fuel, thus presenting a larger surface area of fuel exposed to the air.
Secondary atomisation and initiation. According to the University of California, Los Angeles, studies by A. F. Bush, et ai, picrate microcrystals are formed in the air-fuel mixture due to solvent evaporation. These quickly absorb heat energy and ignite. Further atomisation results and a system of multiple flame fronts develops. This increases the flame speed.
Reducing maintenance, downtime and fuel costs are prime objectives of any efficient operation. Achieving this goal is made increasingly difficult as fuel quality is reduced. Maintenance people need to know more about their fuel specifications so that they can tailor their maintenance to suit. In conclusion, picrate may be unique. It is one product that has been proven beyond doubt to live up to its claims as a combustion improver.
Picrate as Catalysis
As the picrate ignites, iron radicals are released which act as catalytic agents between oxygen in the air and carbon in the fuel. Clearly, the catalytic action promotes oxidation. Similarly, this rapidly assists oxidation of CO to CO2.
Thus the ignition delay is reduced and the period of rapid combustion is assisted by a faster flame speed, more intimate mixing and a more complete burn. The results: peak cylinder pressures are higher, occur earlier and last longer, providing smoother combustion and less mechanical stress. Less desirable fuel components are burnt more cleanly.
Picrate as a catalyst
The following reductions were observed:
Hydrocarbons (HC) 38%
Carbon Monoxide (CO) 12%
Reductions in HCs of 80% and CO of over 50% are regularly recorded in field trials using exhaust gas analysis equipment.
Cleaner oil
Cleaner combustion and cleaner oil go hand-in-hand. High carbon solids (soot) in lube oil is a very common complaint these days, which rapidly overloads the oil’s detergency/dispersancy package. Higher quality oils and more frequent change intervals might appear to be the correct action to take, but is, in fact, a ‘band-aid’ solution to a combustion problem. Picrate is of benefit by directly assisting combustion.
Biological growths
Picrate, also has a strong biocidal activity to effectively control fungal, yeast and bacterial infestations in fuel.
Some results from tests
Reduced engine deposits In an SAE Technical Paper (No.831204), by J B Parsons and G J Germane, the maintenance benefits of additive content picrate were closely examined in several fleets. One 69 piece fleet of Caterpillar equipment included haul trucks, D9 and DI0 dozers, 992 loaders, scrapers and graders. The first engine examined was a 348 Cat from a 992C loader with four months catalyst use, and 8000-meter hours.
Examination of the engine showed carbon deposits at near ‘normal’ levels but with a marked softening of the normal hard carbon being noted in some areas – particularly the centre of the piston crown where bare metal could be exposed by wiping with a rag. There was also evidence of reductions in deposits in the upper liner area above the ring travel, and soot in the manifold exhaust area was “finer and drier”.
A progression of this pattern was observed with engines subsequently overhauled. At the end of the two-year trial, there was almost a complete absence of hard engine carbon, as the soft residue which remained was easily wiped from cylinder heads, valve ports and piston crowns.
The normal build-up of hard scale on these surfaces was absent. Piston rings were exceptionally free of deposits and cylinder compression in high hour engines had been maintained. Valve and piston part numbers were clearly visible. Less exhaust smoke was evident and engine ‘startability’ showed a marked improvement. Of particular interest was the reduction in liner wear being experienced by the haul trucks. At 8000-9000 hours, liners could be reused. This was not typical of previous experience. Fuel efficiency improved by seven per cent.
A thirty-two piece fleet of GM powered buses showed similar maintenance benefits. After 12 months operation on the catalyst treated fuel, the fleet began burning blended fuel with no increase in exhaust emissions, while still maintaining the same level of engine cleanliness.
However, when the catalyst was removed from the fuel, smoke increased to unacceptable levels. Fuel usage reduced 8% due to additive treatment.
Several Cat D353 engines from D9 H dozers in open cut operations were also inspected. They’d operated for some 2800 hours on catalyst treated fuel. Of particular interest was the absence of hard carbon encrustation on the upper ring land of pistons, an area of normal build-up, which often results in bore polishing. Sulphur and hard carbon deposits had vanished from valve faces and exhaust valve stems were exceptionally clean.
The extension to engine life can be quite substantial. Particularly where severe combustion problems (eg bore polishing) have been encountered.


